Clinical Trial Management Course
Clinical Trial Management Course - This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. Learn about regulatory requirements, data analysis, and patient safety. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. Find your path at hmsonline coursestransform your careerleading harvard faculty This course will provide you comprehensive training and develop skills needed to. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. Refer to the data element definitions if submitting registration or results information. To improve attendees’ understanding of related disciplines including research. In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. Gain an overview of principles and practices in clinical research from a global perspective. The aims of the course are: Enroll in sollers college's clinical trials management certification course. To develop attendees’ knowledge and skills in clinical trial management. In this course, you’ll learn about the more advanced elements of managing clinical trials. Learn about regulatory requirements, data analysis, and patient safety. Master clinical trial design and management for medical research. Refer to the data element definitions if submitting registration or results information. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. Find your path at hmsonline coursestransform your careerleading harvard faculty Refer to the data element definitions if submitting registration or results information. To develop attendees’ knowledge and skills in clinical trial management. This course will provide you comprehensive training and develop skills needed to. Enroll in sollers college's clinical trials management certification course. You’ll learn about the computation of sample size and how to develop. You’ll learn about the computation of sample size and how to develop. The aims of the course are: In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation,. This course will provide you comprehensive training and develop skills needed to. Enroll in sollers college's clinical trials management certification course. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. To improve attendees’ understanding of related disciplines including research. In this course,. The aims of the course are: To improve attendees’ understanding of related disciplines including research. You’ll learn about the computation of sample size and how to develop. To develop attendees’ knowledge and skills in clinical trial management. Learn about regulatory requirements, data analysis, and patient safety. You’ll learn about the computation of sample size and how to develop. Find your path at hmsonline coursestransform your careerleading harvard faculty To develop attendees’ knowledge and skills in clinical trial management. Learn about regulatory requirements, data analysis, and patient safety. In this course, you’ll learn more advanced operational skills that you and your team need to run a successful. Learn about regulatory requirements, data analysis, and patient safety. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. Enroll in sollers college's clinical. Gain an overview of principles and practices in clinical research from a global perspective. In this course, you’ll learn about the more advanced elements of managing clinical trials. Enroll in sollers college's clinical trials management certification course. To develop attendees’ knowledge and skills in clinical trial management. Find your path at hmsonline coursestransform your careerleading harvard faculty Find your path at hmsonline coursestransform your careerleading harvard faculty This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. To develop attendees’ knowledge and skills in clinical trial management. To improve attendees’ understanding of related disciplines including research. Enroll in sollers college's. The clinical trials management and regulatory compliance certificate provides rigorous clinical research training across the entire clinical trials process, from the perspective of the clinical. This course will provide you comprehensive training and develop skills needed to. Find your path at hmsonline coursestransform your careerleading harvard faculty To improve attendees’ understanding of related disciplines including research. Explain the concept of. Gain an overview of principles and practices in clinical research from a global perspective. Master clinical trial design and management for medical research. To improve attendees’ understanding of related disciplines including research. Refer to the data element definitions if submitting registration or results information. In this course, you’ll learn about the more advanced elements of managing clinical trials. Gain an overview of principles and practices in clinical research from a global perspective. This course places a strong emphasis on the core principles of clinical trials management, covering essential areas such as site initiation, trial conduct, monitoring, and the effective. This course will provide you comprehensive training and develop skills needed to. Master clinical trial design and management for medical research. From anticipating and planning for protocol events to conducting systematic reviews to synthesize. Enroll in sollers college's clinical trials management certification course. In this course, you’ll learn to collect and care for the data gathered during your trial and how to prevent mistakes and errors through quality assurance practices. Find your path at hmsonline coursestransform your careerleading harvard faculty To develop attendees’ knowledge and skills in clinical trial management. In this course, you’ll learn more advanced operational skills that you and your team need to run a successful clinical trial. To improve attendees’ understanding of related disciplines including research. Learn about regulatory requirements, data analysis, and patient safety. Explain the concept of clinical trial management (ctm) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout. Refer to the data element definitions if submitting registration or results information.What is a Clinical Trial Management Software? Jelvix
Advance Your Career Path Clinical Trial Management Course
ClinEssentials Clinical Trial Manager Training Course YouTube
Clinical Trial Management System (CTMS) PowerPoint and Google Slides
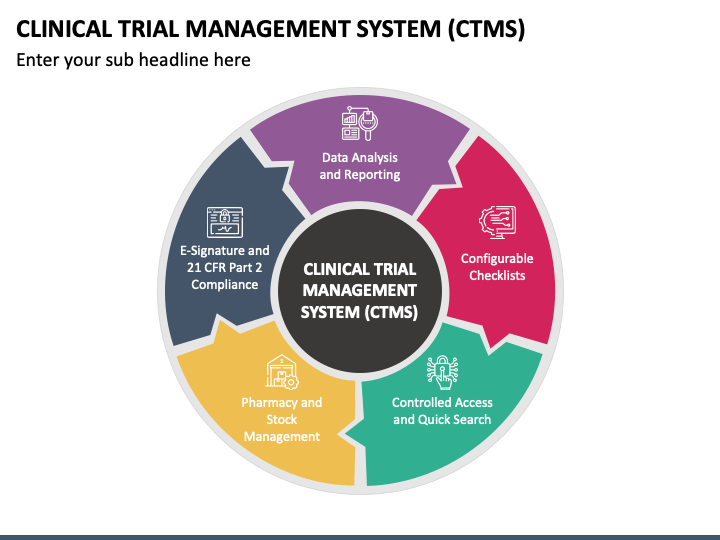
Clinical Trial Management System (CTMS) PowerPoint and Google Slides
Clinical Trial Management System Training Bangalore Clinical Research
Clinical trials management online course starts Feb. 19 University of
PPT Clinical Trial Management Services PowerPoint Presentation, free
PG diploma in Clinical Data Management Course Fees
What is a Clinical Trial Manager (CTM) Salary, Degree Requirements
You’ll Learn About The Computation Of Sample Size And How To Develop.
The Clinical Trials Management And Regulatory Compliance Certificate Provides Rigorous Clinical Research Training Across The Entire Clinical Trials Process, From The Perspective Of The Clinical.
The Aims Of The Course Are:
In This Course, You’ll Learn About The More Advanced Elements Of Managing Clinical Trials.
Related Post: